Why Does Magma Concentration Change During Fractional Crystallization?
Chapter iii Intrusive Igneous Rocks
3.3 Crystallization of Magma
The minerals that make upward igneous rocks crystallize at a range of unlike temperatures. This explains why a cooling magma tin can take some crystals within information technology and withal remain predominantly liquid. The sequence in which minerals crystallize from a magma is known as the Bowen reaction serial (Figure iii.x and Who was Bowen).
Of the mutual silicate minerals, olivine normally crystallizes kickoff, at between 1200° and 1300°C. As the temperature drops, and bold that some silica remains in the magma, the olivine crystals react (combine) with some of the silica in the magma (meet Box 3.1) to form pyroxene. As long as in that location is silica remaining and the rate of cooling is irksome, this process continues down the discontinuous co-operative: olivine to pyroxene, pyroxene to amphibole, and (under the right conditions) amphibole to biotite.
At well-nigh the point where pyroxene begins to crystallize, plagioclase feldspar as well begins to crystallize. At that temperature, the plagioclase is calcium-rich (anorthite) (see Effigy ii.15). Every bit the temperature drops, and providing that there is sodium left in the magma, the plagioclase that forms is a more than sodium-rich diversity.
![Figure 3.10 The Bowen reaction series describes the process of magma crystallization [SE]](https://opentextbc.ca/geology/wp-content/uploads/sites/110/2016/07/Bowen-reaction2-300x150.png)
Who was Bowen, and what'southward a reaction serial?
Norman Levi Bowen, built-in in Kingston Ontario, studied geology at Queen'due south University then at MIT in Boston. In 1912, he joined the Carnegie Institution in Washington, D.C., where he carried out groundbreaking experimental research into the processes of cooling magmas. Working mostly with basaltic magmas, he determined the guild of crystallization of minerals every bit the temperature drops. The method, in brief, was to melt the stone to a magma in a specially made kiln, permit it to absurd slowly to a specific temperature (assuasive some minerals to form), and then quench it (cool it apace) so that no new minerals form (only glass). The results were studied under the microscope and by chemic analysis. This was done over and over, each time allowing the magma to absurd to a lower temperature before quenching.

The Bowen reaction series is ane of the results of his piece of work, and even a century subsequently, it is an important basis for our understanding of igneous rocks. The give-and-take reaction is disquisitional. In the discontinuous branch, olivine is typically the first mineral to form (at but below 1300°C). Equally the temperature continues to drop, olivine becomes unstable while pyroxene becomes stable. The early-forming olivine crystals react with silica in the remaining liquid magma and are converted into pyroxene, something like this:
MgiiSiOfour + SiOii ——> 2MgSiO3
olivine pyroxene
This continues downwards the chain, as long as there is nevertheless silica left in the liquid. [prototype from Wikipedia: http://en.wikipedia.org/wiki/File:NormanLBowen_1909.jpg ]
In cases where cooling happens relatively apace, individual plagioclase crystals tin can be zoned from calcium-rich in the center to more sodium-rich around the outside. This occurs when calcium-rich early-forming plagioclase crystals become coated with progressively more sodium-rich plagioclase as the magma cools. Figure 3.eleven shows a zoned plagioclase under a microscope.

Finally, if the magma is quite silica-rich to begin with, in that location will still be some left at effectually 750° to 800°C, and from this terminal magma, potassium feldspar, quartz, and perhaps muscovite mica will course.
The limerick of the original magma is critical to magma crystallization considering information technology determines how far the reaction process tin proceed before all of the silica is used up. The compositions of typical mafic, intermediate, and felsic magmas are shown in Figure three.12. Note that, different Figure iii.6, these compositions are expressed in terms of "oxides" (e.g., Al2O3 rather than just Al). There are ii reasons for this: i is that in the early belittling procedures, the results were always expressed that style, and the other is that all of these elements combine readily with oxygen to class oxides.
![Figure 3.12 The chemical compositions of typical mafic, intermediate, and felsic magmas and the types of rocks that form from them. [SE]](https://opentextbc.ca/geology/wp-content/uploads/sites/110/2016/07/mafic2-300x168.png)
Mafic magmas accept 45% to 55% SiO2, almost 25% total of FeO and MgO plus CaO, and about 5% NatwoO + K2O. Felsic magmas, on the other manus, have much more SiO2 (65% to 75%) and Na2O + K2O (around 10%) and much less FeO and MgO plus CaO (virtually five%).
Exercise 3.3 Rock Types Based on Magma Composition
The proportions of the chief chemical components of felsic, intermediate, and mafic magmas are listed in the table beneath. (The values are like to those shown in Figure 3.12.)
| Oxide | Felsic Magma | Intermediate Magma | Mafic Magma |
|---|---|---|---|
| SiO2 | 65-75% | 55-65% | 45-55% |
| Al2O3 | 12-16% | 14-xviii% | 14-18% |
| FeO | two-4% | 4-8% | 8-12% |
| CaO | 1-4% | 4-vii% | vii-11% |
| MgO | 0-iii% | two-half dozen% | 5-nine% |
| Na2O | 2-6% | iii-7% | one-three% |
| Grand2O | iii-5% | 2-iv% | 0.five-3% |
Chemical data for 4 stone samples are shown in the following table. Compare these with those in the tabular array above to determine whether each of these samples is felsic, intermediate, or mafic.
| SiO2 | AltwoOthree | FeO | CaO | MgO | NaiiO | K2O | Type? |
|---|---|---|---|---|---|---|---|
| 55% | 17% | 5% | 6% | iii% | iv% | 3% | |
| 74% | 14% | 3% | iii% | 0.5% | 5% | 4% | |
| 47% | fourteen% | 8% | 10% | 8% | 1% | 2% | |
| 65% | fourteen% | four% | 5% | 4% | iii% | 3% |
As a mafic magma starts to absurd, some of the silica combines with iron and magnesium to make olivine. As information technology cools further, much of the remaining silica goes into calcium-rich plagioclase, and any silica left may be used to catechumen some of the olivine to pyroxene. Before long later that, all of the magma is used up and no further changes takes place. The minerals present will be olivine, pyroxene, and calcium-rich plagioclase. If the magma cools slowly secret, the product volition be gabbro; if information technology cools chop-chop at the surface, the production will be basalt (Figure three.xiii).
Felsic magmas tend to be cooler than mafic magmas when crystallization begins (because they don't have to be every bit hot to remain liquid), and and then they may start out crystallizing pyroxene (non olivine) and plagioclase. Equally cooling continues, the various reactions on the discontinuous branch will proceed because silica is arable, the plagioclase will become increasingly sodium-rich, and somewhen potassium feldspar and quartz volition form. Commonly even very felsic rocks will not accept biotite or muscovite because they may not have enough aluminum or plenty hydrogen to brand the OH complexes that are necessary for mica minerals. Typical felsic rocks are granite and rhyolite (Figure 3.xiii).
The cooling behaviour of intermediate magmas lie somewhere betwixt those of mafic and felsic magmas. Typical intermediate rocks are diorite and andesite (Effigy 3.xiii).
![Figure 3.13 Examples of the igneous rocks that form from mafic, intermediate, and felsic magmas. [SE]](https://opentextbc.ca/geology/wp-content/uploads/sites/110/2016/07/igneous-rocks2-300x165.png)
A number of processes that take place within a magma sleeping accommodation tin can affect the types of rocks produced in the end. If the magma has a low viscosity (i.e., it's runny) — which is probable if information technology is mafic — the crystals that form early, such as olivine (Figure iii.14a), may slowly settle toward the bottom of the magma chamber (Figure iii.14b). The ways that the overall composition of the magma near the acme of the magma chamber volition become more than felsic, as information technology is losing some iron- and magnesium-rich components. This process is known as fractional crystallization. The crystals that settle might either form an olivine-rich layer nearly the bottom of the magma bedroom, or they might remelt because the lower part is likely to exist hotter than the upper office (think, from Chapter 1, that temperatures increase steadily with depth in Earth because of the geothermal slope). If any melting takes place, crystal settling volition make the magma at the bottom of the chamber more than mafic than information technology was to begin with (Figure 3.14c).
![Figure 3.14 An example of crystal settling and the formation of a zoned magma chamber [SE]](https://opentextbc.ca/geology/wp-content/uploads/sites/110/2016/07/magma-chamber2-300x188.png)
If crystal settling does not take place, because the magma is too viscous, then the process of cooling volition proceed as predicted by the Bowen reaction serial. In some cases, nonetheless, partially cooled but notwithstanding liquid magma, with crystals in information technology, will either motility farther up into a cooler office of the crust, or all the way to the surface during a volcanic eruption. In either of these situations, the magma that has moved toward the surface is likely to cool much faster than information technology did inside the magma sleeping accommodation, and the rest of the rock volition have a finer crystalline texture. An igneous rock with large crystals embedded in a matrix of finer crystals is indicative of a ii-stage cooling procedure, and the texture is porphyritic (Figure 3.15).
![Figure 3.15 Porphyritic textures: volcanic porphyry (left – olivine crystals in Hawaiian basalt) and intrusive porphyry (right) [SE]](https://opentextbc.ca/geology/wp-content/uploads/sites/110/2016/07/porphyry-300x114.png)
Practise iii.4 Porphyritic Minerals
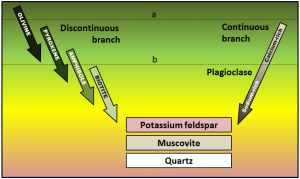
Equally a magma cools below 1300°C, minerals start to crystallize within it. If that magma is then involved in a volcanic eruption, the rest of the liquid will cool quickly to class a porphyritic texture. The rock will have some relatively large crystals (phenocrysts) of the minerals that crystallized early, and the rest will exist very fine grained or even glassy. Using the diagram shown here, predict what phenocrysts might exist present where the magma cooled as far as line a in one example, and line b in another.
Source: https://opentextbc.ca/geology/chapter/3-3-crystallization-of-magma/
Posted by: bellgunfoop.blogspot.com

0 Response to "Why Does Magma Concentration Change During Fractional Crystallization?"
Post a Comment